Ι The Box Turtles Project Ι The Ephemeral Pools Project Ι The Lizards Project Ι The Semi-aquatic Turtles Project Ι The Snakes Project Ι
The Stream Amphibians Project
 Written by:
Written by:
Andrew Ash, Catherine Matthews and Lacey Huffling
Before starting a project similar to the one described in this curriculum, contact your state wildlife resources commission or state division of fish and game to see what kinds of permits you need to work with animals.
This curriculum project focuses on amphibians that live in small North Carolina streams in the piedmont and in the inner coastal plain. It also investigates other organisms that are denizens of such streams and the food chains that are formed by the interactions of these creatures. This study was conceived and developed by The HERP (Herpetology Education in Rural Places and Spaces) Project staff. The following curriculum is meant to support your efforts to develop your own educational program about small-order NC streams or other similar ecosystems. We provide information about the animals and food chains that are commonly found in such streams, the materials and methods we use to study them, and suggestions for data collection and reporting.




After completing this project, participants will be able to:
- Define the term macroinvertebrate and list and identify several common macroinvertebrates in streams (dragonfly nymphs, damselfly nymphs, water striders, water scorpions, etc.)
- Explain the trapping methods that are used in this project (leaf packs and minnow traps)
- Discuss the threats to small streams in the geographical region where the streams are located
- List characteristics of salamanders
- Identify salamanders to family (and with adults, to species)
- Compare and contrast the life cycles of frogs and salamanders
Small streams that are wide enough to jump over are very common in North Carolina and contain a surprising number and diversity of animals that form food chains of remarkable complexity. Amphibians are often dominant members of such communities.
Amphibians were the first major group of vertebrates (animals with backbones) to exist on land. For that reason, their bodies and life cycles are in many cases transitional between fully aquatic fish and fully terrestrial reptiles, birds and mammals. Listed below are some important characteristics of modern amphibians:
- A glandular, moist skin that is susceptible to drying and the absorption of noxious or toxic substances (in contrast, reptiles have scales)
- Lack of an external earhole (present in reptiles)
- Lack of nails/claws (found in reptiles)
- A vent opening that is oriented longitudinally with the body (as opposed to transversely in reptiles)
- Most forms in North Carolina have four limbs, but these may be reduced or partially absent in aquatic forms
- Soft gelatinous eggs with no hard protective covering such as an egg shell. These eggs are laid in water or moist terrestrial environments.
- Life cycles with or without an aquatic larval stage

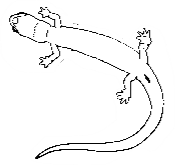
Many people confuse salamanders and lizards. The five-lined skink, a lizard (with an external ear hole, claws, etc.) photograph below on left, and the three-lined salamander, photograph above right, are often confused.
In piedmont streams we have studied, we often catch frogs and salamanders. The herps below are commonly found and you can get additional information about each animal by going to the Herps of North Carolina website.
- Bullfrog (Lithobates catesbeiana) http://www.herpsofnc.org/herps_of_NC/anurans/Rancat/Ran_cat.html
- Green Frog (Lithobates clamitans) http://www.herpsofnc.org/herps_of_NC/anurans/Rancla/Ran_cla.htm
- Pickerel Frog (Lithobates palustris) http://www.herpsofnc.org/herps_of_NC/anurans/Ranpal/Ran_pal.html
- Southern Leopard Frog (Lithobates sphenocephala) http://www.herpsofnc.org/herps_of_NC/anurans/Ransph/Ran_sph.html
- Northern Dusky Salamander (Desmognathus fuscus) http://www.herpsofnc.org/herps_of_NC/salamanders/Descon,%20fus/Des_con,%20fus.html
- Mud Salamander (Pseudotriton montanus) http://www.herpsofnc.org/herps_of_NC/salamanders/Psemon/Pse_mon.html
- Red Salamander (Pseudotriton ruber) http://www.herpsofnc.org/herps_of_NC/salamanders/Pserub/Pse_rub.html
- Three-lined Salamander (Eurycea guttolineata) http://www.herpsofnc.org/herps_of_NC/salamanders/Eurgut/Eur_gut.html
- Southern Two-lined Salamander (Eurycea cirrigera) http://www.herpsofnc.org/herps_of_NC/salamanders/Eurcir/Eur_cir.html
In streams in the inner coastal plain a few additional species of salamanders can be found:
- Amphiuma (Amphiuma means) http://www.herpsofnc.org/herps_of_NC/salamanders/Ampmea/Amp_mea.html
- Siren (Greater Siren (Siren lacertina) and Lesser Siren (Siren intermedia)) http://www.herpsofnc.org/herps_of_NC/salamanders/Sirlac/Sir_lac.html http://www.herpsofnc.org/herps_of_NC/salamanders/Sirint/Sir_int.html
Among other organisms, crayfish and many species of adult and larval insects can be found. Finfish are not overly common in our streams, but they may be very common in slightly larger watercourses. Unfortunately, small watercourses can be fragile, and often are easily polluted by chemicals, siltation, light or heat. Lack of any or all of the organisms mentioned above is a good indicator that stream conditions are not optimal.
The exact species you encounter will depend on where you live, and on the size and environmental quality of the stream you use. Good field guides will be indispensable to this particular project.
While doing fieldwork in North Carolina, participants may encounter insects and other arthropods including chiggers, yellow jackets, ticks and spiders. Using insect repellant (but not on your hands if you plan to collect herps) and wearing a hat and long pants are useful ways of preventing these animals from biting you, stinging you or attaching to you. Pulling one’s socks over the bottoms of pants legs is an especially good way of preventing ticks, chiggers, and spiders from crawling up your legs. Participants should also wear sunscreen and carry water; sturdy boots are useful when hiking in rough terrain. If you are in an area where you may encounter snakes, know that feet and ankles are the most common bite locations, followed by hands. Wear protective footwear and long pants or gaiters and look before you place your hands down or around a tree. Always hike with a partner and let someone else know your itinerary.
Care should be taken when working around water if learners are inexperienced swimmers. Leaders also should be mindful of the dangers associated with the various sorts of aquatic reptiles and amphibians. Certain amphibians and invertebrates can deliver a healthy bite or pinch; first-timers not familiar with these organisms may choose to wear heavy gloves. Upon return from the field, all individuals should wash their hands immediately. In the unusual event of a serious bite or sting, medical assistance should be sought immediately even if it means termination of the activity.
General. When handling any live animal, it is important to keep two safety issues in mind: the safety of the person who is holding the animal and the safety and welfare of the animal itself. The humane treatment of any animal in field research is both an ethical and a scientific necessity. Amphibians are especially susceptible to insult from any noxious chemical (poisons, insecticides, strong household cleansers and the like) and/or living organisms (bacteria, fungi or other microbes) due to their moist, permeable skin. Any person intending to handle amphibians should wash and rinse their hands thoroughly with antibacterial soap and clean water, making sure to wash each time before picking up a new animal so as to avoid cross-contamination. Amphibians must be kept hydrated at all times. If their skin becomes sticky or dry, it is time to release the animal or irrigate it with natural water (i.e., water from its habitat). This is especially true of small or larval amphibians that don’t carry a large reserve of water in their bodies and can desiccate quickly. Tap water from city reservoirs, often called “city water”, or other chemically treated water (including bottled water) should never be used to hydrate amphibians. Additionally, amphibians can have lightly ossified skeletons that are not resistant to strong pressures or forces so they must be handled with care.
Trapping. This curriculum describes a trapping technique. The interval between setting traps and checking traps (active traps such as minnow traps from which animals cannot escape), should be as short as possible, never more than 24 hours. A set trap is a potential death sentence for the animal to be trapped and it is the researcher’s ethical imperative to check traps in a timely manner. Lead instructors/investigators must make every effort to prevent trap deaths from exposure or drowning. The traps should be positioned using some type of flotation device so that captured amphibians without gills have access to air. We make sure that each of our traps floats before we leave our study site.
Although most amphibians in North Carolina cannot deliver a harmful bite, there are one or two, such as amphiumas in the coastal plain, that can. Additionally, stream invertebrates such as crayfish or insects with piercing mouthparts can deliver a painful pinch or bite, and may draw blood. All participants, both instructors and students, should be prepared for the possibility of being bitten or pinched by these animals as they seek to defend themselves. Keep fingers and hands out of range of pincers and mouth parts at all times. Crayfish can be handled by grasping them with thumb and pointer finger on their thorax directly behind their pincers.
Holding & NOT Holding amphibians and invertebrates. These animals must always be handled gingerly. This can be a large problem with frogs since they have strong muscular legs that allow them escape a captor’s hands. A good way to hold a frog without damage is to extend the legs and hold them gently but firmly while supporting the body of the frog with the other hand. As long as the frog cannot cock its legs, it will have a hard time jumping.

The two photographs above show how to restrain the rear legs of the frog so it is unable to escape a captor’s hands.
Adult salamanders can be held with thumb and pointer finger; the thumbs should be placed on their pectoral region between and just posterior to the front legs, with the pointer finger on their back just opposite the thumb.
A Video of Holding and Identifying a Frog
These suggestions do not apply to eel-like aquatic salamanders such as amphiumas and sirens. We do not handle these organisms during our lessons, and only an experienced professional should handle them. Larval amphibians are delicate creatures; we do not handle them, and suggest that they not be handled. Instead, we place them in buckets of natural water for inspection and identification. Likewise, we do not handle aquatic insects; rather we place them in buckets for close examination.
A Video of a Two-Toed Amphiuma
Processing animals and collecting data. Keep all amphibians out of the direct sun so they will not dry out or overheat. Amphibians are ectotherms, or animals that derive body heat from external sources. Usually they avoid overheating in their natural environments by immersing themselves in water, burrowing in the soil, or seeking refuge under leaf litter. Try not to hold them in your hands for too long. As mentioned above, amphibians must remain hydrated during processing.
Return all amphibians and insects to their natural habitats as soon as possible. Excessive handling and confinement stresses these animals in many ways. It is important to return each animal to the exact place where it was found. When examining nature, a cardinal rule is to leave the system exactly as you found it, to the greatest extent possible. In general, keep the following guidelines in mind:
- All animals must be released at the point of capture.
- Animals should be processed quickly and efficiently with minimal handling.
- Almost all animals can bite. Some rarely do, and some do so without hesitation. Know your species!
- Hands should be washed both before and after handling animals.
- GPS recorder
- An android equipped with the appropriate data collection app (this app is The HERP Project app, available as a free download at theherproject.uncg.edu). If androids are not available, paper datasheets are fine.
- Metric rulers
- Metric thermometers
- A humidity gauge
- Calipers 0–6 in., (0-150mm)
- pH paper
- Several five gallon industrial food containers (can be bought at your local building supply store)
- Sorting trays –white porcelain shallow pans are excellent – we order our from Forestry Suppliers (http://www.forestry-suppliers.com, stock # 359)
- Wildlife meshing of approximately 1 inch net gage (We use bird netting from the Garden section at Lowe’s (http://www.lowes.com/pd_510599-23132-208513_0__?productId=50119481&Ntt=bird+netting&pl=1¤tURL=%3FNtt%3Dbird%2Bnetting&facetInfo=, but orange bags or onion bags could work as long as the mesh gauge is about 1 inch to let in adult salamanders)
- Twist ties
- Local leaves and twigs
- Minnow Traps (Funnel-type)
- Bait for minnow traps (we use sardines in cans)
- Empty plastic bottles (to keep the minnow traps afloat)
- Surveyor pins
- A pictorial key to aquatic insect orders (we use printed and laminated copies of the North Carolina macroinvertebreate keys. These are included in this document after The Herp Project Data Sheet and are available for a free download):
http://www.learnnc.org/lp/media/uploads/2010/06/macrokey.pdf
http://www.ncparks.gov/Education/docs/lano_eele.pdf (on page 3.1.8 and 3.1.9)
- A field guide to the amphibians local to your area (we use Amphibians & Reptiles of the Carolinas and Virginia (2010).)
Some things to think about:
- Why do amphibians need water in their environment?
- What factors determine who eats whom in a stream food chain?
- What is a life cycle?
- Why are streams important to many species of larval and adult amphibians?
Our piedmont stream amphibian exercise utilizes six sampling stations spread along approximately 200 meters of streambed. The sites were deliberately set up to examine the impact of sunlight and heat on the first two stations. The number and positioning of the stations should reflect the environmental issues you are addressing and the personnel you have at your disposal. The total sampling time for our six stations is approximately three hours, which includes 20 minutes of general introduction and another 30 minutes of debriefing, summary and data transfer. The project is designed such that data can be recorded on hard copy datasheets, which are supplied to each student, or entered into The HERP Project’s android app that can upload information via the Internet.
During the introductory period, students will notice general environmental conditions (rain, cloud cover, wind, air temperature and relative humidity).
At this point, the students will be introduced to the general sampling scheme at each station. There are a total of three traps of two different kinds at each station. One trap is a leaf pack and the other two are minnow traps. Leaf pack traps are made by us, and the minnow traps (the funnel type) are commonly available at fishing or outdoor supply stores. The photograph below shows a leaf pack trap in a sorting tray.
Leaf pack traps. Leaf pack traps should be installed 4-8 days in advance of sampling. Animals may come and go as they please in leaf pack traps after they are set; thus they are passive traps. This condition is in contrast to that of minnow traps, which are active traps. Once animals have entered minnow traps they are typically unable to escape until the traps are opened. If you will be sampling on five successive days, for instance, five leaf packs will need to be placed at each station so that one can be checked each day. To create the leaf pack traps, obtain several garbage bags full of hardwood leaf litter from the forest near your stream. Wrap a double handful of leaves into an 18-inch square of wildlife meshing. Close bundle with a twist tie and you are done.
The photograph on the left is of a student carefully starting to sort a recently removed leaf pack. Note the white sorting trays and how they provide contrast against the dark leaf matter the student will be sifting through to locate stream amphibians and macroinvertebrates. A thin layer of water has been poured into the sorting dish to keep the organisms moist while the students process the trap.
To hold them in place and under the water’s surface we use long, red and white surveyors’ pins threaded through the leaf packs. Because the species, including amphibians, that are attracted to the leaf packs do not breathe air exclusively (they can obtain oxygen from the water) and can escape if necessary, no harm can come from their prolonged submersion. We have caught a variety of insects and both larval and adult salamanders in such packs and they are unharmed by the capture method.

Minnow Traps. One minnow trap should be baited with a smelly food item; we use sardines. The can of sardines is punctured so that the fluids can escape but the fish stay in the can. The other trap should remain unbaited to serve as a control. Or, one trap can include a light source, like a glowstick, and the other, paired trap would have no glowstick. This gives instructors the opportunity to discuss the necessity/value of controlled experiments in science.
Minnow traps should be baited and placed in the stream the afternoon before survey. If surveys are to occur on successive days, traps can be inspected, re-baited and immediately placed back in the water, thus allowing a maximal trapping period for the next day. Minnow traps should be placed such that the entrance holes on either end are under water, but so the trap protrudes into the air at some point along its length to allow trapped animals access to air. Unlike the leaf pack trap, the minnow traps may capture amphibians or other species that must have access to atmospheric oxygen. If the stream is too deep, plastic bottles can be used as floats to keep at least some of the trap above water.
A Video of Traps and an Experimental Design
A Video of a Minnow Trap
The photographs are examples of minnow traps. Notice the plastic bottles in the traps. These are used to float the trap to ensure that trapped organisms do not drown before the trap is checked.
At each station students will collect air and water temperature, and pH of the water. Once this is done, a leaf pack will be extracted from the stream and carefully inspected for insects or amphibians. We use white sorting trays filled with enough stream
 water to cover the bottom. The sorting trays provide good contrast for the darker organisms. This sorting process can be slow, so students need appropriate oversight/encouragement. Numbers of insects of various orders and the species of amphibians (adults and larvae) should be recorded if identification is possible. Length of adult salamanders should be recorded from the tip of the snout to the posterior terminus (end) of the vent (this is called a Snout Vent Length measure or SVL) in mm. SVL is used because salamanders can lose their tails to predators and regenerate them, so SVL provides a more consistent indicator of animal size. The drawing on the right indicates where the vent is located on salamander. Diagram retrieved from http://www.pwrc.usgs.gov/amphib/primenet/shenterrestrial.html
water to cover the bottom. The sorting trays provide good contrast for the darker organisms. This sorting process can be slow, so students need appropriate oversight/encouragement. Numbers of insects of various orders and the species of amphibians (adults and larvae) should be recorded if identification is possible. Length of adult salamanders should be recorded from the tip of the snout to the posterior terminus (end) of the vent (this is called a Snout Vent Length measure or SVL) in mm. SVL is used because salamanders can lose their tails to predators and regenerate them, so SVL provides a more consistent indicator of animal size. The drawing on the right indicates where the vent is located on salamander. Diagram retrieved from http://www.pwrc.usgs.gov/amphib/primenet/shenterrestrial.html
 Leaves may be discarded on the forest floor and meshing placed in a garbage bag for later disposal. Careful record keeping will assure results are accurate. Be sure to note if minnow traps are baited or unbaited and if and when they have been inspected for amphibians and other animal life. Captured individuals should be identified to species if possible and measured as above. Crayfish are commonly caught in minnow traps, and they can be quickly discussed and released. Students enjoy sexing crayfish and watching them move through the water. Finfish should be treated in the same manner as crayfish. For all organisms captured, numbers of individuals should be counted, with larval forms being a separate category.
Leaves may be discarded on the forest floor and meshing placed in a garbage bag for later disposal. Careful record keeping will assure results are accurate. Be sure to note if minnow traps are baited or unbaited and if and when they have been inspected for amphibians and other animal life. Captured individuals should be identified to species if possible and measured as above. Crayfish are commonly caught in minnow traps, and they can be quickly discussed and released. Students enjoy sexing crayfish and watching them move through the water. Finfish should be treated in the same manner as crayfish. For all organisms captured, numbers of individuals should be counted, with larval forms being a separate category.
Reporting Data to The HERP Project & The Carolina Herp Atlas
Through use of the free Herp Project android application (available for FREE download, see below!), HRE participants record data and upload it to an open source database found on the Herp Project website. This enables us to compare our data with previous years, and we can download data sets for further analysis. We also report our data to the Carolina Herp Atlas, a citizen science database initiated to document the distributions of amphibians and reptiles across North and South Carolina.

Students examine a crayfish, as they begin to contemplate food chains and energy flow dynamics of local streams.
Once all stations have been surveyed, the group can retreat to a comfortable spot to review what has been achieved. We have found it useful to discuss these ecosystems from a food chain/energy flow perspective as well as interpreting sources of environmental degradation for each ecosystem.

Photograph of one of the Piedmont surveys our stream amphibian project monitors.
The exact species you encounter will depend on where you live, and by the size and environmental quality of the stream you use. Good field guides will be indispensable to this particular project.
Small streams in the piedmont are common resources and have ecological value at many levels. They feed water to larger rivers and streams. They are associated with wetland vegetation and provide habitat for animals that require moist soil or access to water. Streams and associated wetlands provide topographic variety that enhances both plant and animal diversity at a regional level. In North Carolina, the piedmont is the most heavily populated area of the state, and such habitats are increasingly being altered into urban watercourses that feed into sewage/stormwater systems. They may become part of neighborhood or agricultural landscapes where they are exposed to excessive sunlight and heat. Pollution and/or environmental degradation of such areas will affect not only the immediate site, but downstream waters and ecosystems as well. Our piedmont streams contain an abundance of life that is overlooked by most and are among the ecosystems that are most in need of conservation.
Beane, J. C., Braswell, A. L., Mitchell, J. C., Palmer, W. M., & Harrison, J. R. (2010). Amphibians & Reptiles of the Carolinas and Virginia. Chapel Hill, NC: The University of North Carolina Press.
Dorcas, M. E., Price, S. J., Beane. J. C., & Cross, S. S. (2007). The Frogs and Toads of North Carolina: Field Guide and Recorded Calls. Raleigh, NC: North Carolina Wildlife Resources Commission.
Dorcas, M. E., Price, S. J., Beane. J. C., & Cross, S. S. (ND). The Frogs and Toads of North Carolina. Retrieved from http://www.herpsofnc.org/herps_of_NC/anurans/anurans.html
North Carolina State University (ND). NC State AgNIC systematic entomology: A guide to online insect systematic resources. Retrieved from http://www.lib.ncsu.edu/agnic/sys_entomology/id/keys.html
Petranka, J. W. (1998). Salamanders of the United States and Canada. Washington: Smithsonian.
Powell, R., Collins, J. T., & Hooper, E. D. (1998). A Key to Amphibians and Reptiles of the Continental United States and Canada. Lawrence: University Press of Kansas Press.
United States Geological Survey (2001). Restraint and handling of live amphibians. Retrieved from http://www.nwhc.usgs.gov/publications/amphibian_research_procedures/handling_and_restraint.jsp
Visual Learning Software. VL HERPS is a free visual learning software program designed for learning reptiles and amphibians of the Southeast at home. http://theherpproject.uncg.edu/visual-learning-software/
 This project is supported by the National Science Foundation, Grant No. DRL-1114558. Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation.
This project is supported by the National Science Foundation, Grant No. DRL-1114558. Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Transcript of Holding and Identifying a Frog:
Andy – Ok, as I’ve said before, I find the easiest way to hold frogs is by the very end of their rear, or hind, legs so that they can’t cock to jump. So, what we want to do is identify this so everybody get their field guides out and we’re going to look for the characters that we discussed earlier and y’all are going to tell me what I’m looking at here.
Participant – Well, you’ve got that lateral, or ridge, what’s it called again?
Andy – Lateral line, lateral fold.
Participant – That goes down behind its ears.
Andy – Behind its ears. There’s one clue.
Erika – Do you remember what it’s called?
Participant – Tympanum?
Erika – Tympanum, yes!
Andy – Ok, so, do we see any brightish or neon green up under its eye. So what do you think, a frog this big, what do you think our two choices are?
Participants – Green frog or Bullfrog.
Andy – Green frog or Bull so do we see that neon green under the eyes that Green frogs have? Now Bulls can sometimes look like that too.
Participant – Not really.
Andy – I don’t really see that. It’s kinda green but not really neon green.
Participant – Um, 145 and 146.
Andy – So, the real key in this case is what that fold does. And what have we decided it does?
Participant – Curves back around behind the ear so I say it’s an American Bullfrog.
Andy – There you go. And let’s look at his stomach. So you see the splotching, the darker splotching on the venter, right? Ok, so that pretty much tells us. Ok, so now let’s look at the size of the tympanum and y’all tell me whether we’re looking at a young man or a young lady here.
Participants – Female. They’re small in size.
Andy – Yeah, smaller tympanum. Ok, so, anyone want to hold the frog?
Erika – Let’s show them the eyeball. Ok, look at his eyes, guys. See, I can push it back, see how I can just push it down in?
Participant – Does that…
Andy – Oh they have muscles that can actually pull their eyeballs in and out. So why would you want your eyeballs up like that?
Participants – So you can see above the water?
Andy – Yeah, so you can stay in the water but see what’s above the water. But why would you want to be able to pull them down?
Participants – So predators don’t see you.
Andy – Predator… let’s think about some other things. How do you reckon frogs find a lot of their food?
Participants – Under the water… Oh so they can sneak up on them.
Andy – Well, they’re gonna be nosing around in litter right? Pushing through leaves and sticks and twigs. Do you want your eyes up where they can be damaged doin that kind of thing?
Participant – Uh uh.
Andy – No, you want to pull them down. And most amphibians depend on their sense of smell as their primary sense. Ah, I work on salamanders and I had a salamander physiologist from Germany once tell me that salamanders are just giant noses. They can see but they don’t use their eyes much. And salamanders can do the exact same thing, they have telescope eyes that they can pull down when they’re foraging under leaves or under rocks in streams and things like that.
Participant – May I hold him?
Andy – Sure, so just grab right there at the end. There you go.
Participant – Oh, ok. I thought he would be more jumpy.
Andy – No, you see, if he can’t cock his legs, there’s not much he can do. Or she, excuse me, that’s a young lady.
Participant – There were three tadpoles, correct?
Participant – Four. Four small ones.
Erika – You want to try picking one up and you can see what it is?
Participant – Any of them have legs?
Andy – Do you think Green frog adults would eat Bullfrog tadpoles?
Participant – Sure.
Andy – Sure. Would an adult Bullfrog eat a Green frog or a Green frog tadpole? Sure.
Transcript of Two-Toed Amphiuma:
Erika – So what does it look like to you guys?
Participants – Eels.
Erika – It looks like an eel?
Participant – Uh huh.
Erika – But what is it really?
Participant – Salamander.
Erika – It’s a salamander. So these are the largest salamanders in North America.
Leader – They can reach sizes of up to 48 inches. This is a pretty good guy here. As you can see, well, I don’t know if you can see, but right here, see that little appendage right there?
Participant – Yeah.
Leader – With two little itty bitty toes? Can y’all see that?
Participant – Yeah.
Leader – Can everybody see that?
Erika – He’s got two back here and two up front.
Leader – See that little foot right there? That little itty bitty foot?
Erika – And this guys called a two-toed amphiuma. So like I was saying earlier, he’s got six rows of teeth and they’re all segmented so they’re broken up, so he may have two on this side, two on this side, and two in the middle but they’re all broken up.
Leader – So what would you think that these guys eat?
Participant – Small fish.
Leader – Small fish, yeah. What else would live back in this area that they might like to eat?
Participant – Frogs.
Leader – Frogs? Yeah. With six rows of teeth they can pretty much eat anything, right? Their main forage is usually crayfish. You know, crayfish have shells but it’s nothing for them to crunch through those shells with six rows of teeth.
Erika – These guys are mostly active at night. When there’s heavy, when there’s a lot of heavy rain they can cross over roads to the other side and they’ll go to, like, a ditch or something.
Leader – How do you think these guys breath?
Participant – Lungs.
Leader – Lungs? Maybe, anybody else got an idea?
Participant – Skin.
Leader – Skin… What do you think, Emily?
Emily – I don’t know.
Leader – You don’t know?
Emily – No.
Leader – These guys can actually breathe all three ways: they can breathe through their skin, they also have a lung so they can gulp air and breathe like we do, and they also have internal gills. So they’re kinda the ultimate survivor, they can survive in just about any kind of environment.
Erika – So not a lot is known about their early stages of life because not a lot of people catch these things. They’re really abundant in areas but they’re just not caught a lot. So a lot isn’t known about their early stages of life. We do know they can lay anywhere from 10 up to 200 eggs and they usually lay in, like, damp places.
Transcript of Traps and Experimental Design:
Andy – So, at each site in this particular project, we have three different kinds of traps. One of them is a leaf-pack trap and that’s pretty much a passive trap where we put the packs in the water and macroinvertebrates and certain kinds of salamanders and amphibians will crawl in there for cover during times of the day when they’re not active. And then they can hide in there and so then we can pull those little packs out and see what we’ve got.
In addition, at each site, we have two minnow traps, one of which we bait and we bait with sardines, but you’re free to try whatever you’d like, and the other minnow trap is unbaited. We can attract a variety of different kinds of things in those baited and unbaited traps. We regularly get crayfish, we get adult and tadpole frogs, we get adult and larval salamanders in those sorts of traps. So we can talk a little bit about food chains and who eats who and energy processing and stuff like that. Mostly we try to emphasize the numbers of animals that you can find in streams of this size that you can jump across.
We can then talk about experimental design, controlled experiments and why controls are important. Then we let the students think a little about which of our minnow traps has a manipulative variable and which of it has a control, why those work the way they do. And the interesting thing about it is we get varied results on that, our results are not necessarily clear cut. The main conclusion that I have come to over three or four years is that frog tadpoles seem to be attracted to baited traps and I can’t tell much difference between any of the other animals. They seem to go into the other two kinds of traps almost equally.
Ok, so that’s a little bit about experimental design.
Transcript of Minnow Traps:
Erika – You’ve seen them? So you guys know what they look like?
Participant – Yeah.
Erika – So who wants to give me a brief description? Go ahead Emily.
Emily – I don’t know, they can swim into it but they can get out.
Erika – What shape does it tend to be?
Participant – Like a cone.
Erika – Like a cone? Ok, so, why can they swim in and not out?
Emily – The shape of it…
Participant – It kinda funnels them in.
Erika – It funnels in, that’s right.
So this is what your basic minnow trap is gonna look like. I know that they have different sizes for these, you can get ones with bigger holes and ones with smaller holes. What do you notice that we put in all our traps that you’ve seen?
Participant – A bottle so it floats.
Erika – We put a bottle so it floats. Why do we want it to float?
Emily – So that they can get air.
Erika – So they can get air? Ok.
Participant – So it’s not sitting on the bottom.
Erika – So it’s not sitting on the bottom, ok. And we don’t want anything in here that needs air to drown, right? Ok so when you set a trap like this, how often do you want to check it?
Participant – Every day.
Erika – Everyday, how many times a day do you think?
Participant – Probably twice.
Erika – Once or twice a day, why do we want to do that?
Participant – To make sure that, if you do have something in there that you take it out.
Erika – Ok, that’s right.
Ok, what sorts of things do you think we would catch in this? What kind of amphibians? Or maybe not even amphibians, other things too.
Participant – Salamanders.
Erika – Salamanders, ok.
Participant – Amphiumas.
Erika – Amphiumas, ok. Anybody else? What do we hear calling every night?
Participants – Frogs.
Erika – Frogs, ok. And then what is this thing, it would normally be alive?
Participant – Fish.
Erika – A fish. So fish, what other kinds of things could we find in here?
Participant – Snakes.
Erika – Ok, snakes. And what is this thing here?
Emily – A spider.
Erika – Spiders, ok, so we can find bugs. So there’s a variety of things you can find in here and there are a bunch of different ways that you could bait your traps. You can put sardines like we did, we stole your idea Christine. So you can put sardines or you can put chicken, we put chicken in one. Last year we did glow sticks. So you can use a bunch of different kinds of things to try…
Participant – Glow sticks?
Erika – Mm hmm, we were trying to see if the light attracted…
So this is going to be what your basic minnow trap will look like so, when you’re done, and you don’t see anything in it. You normally wanna roll it around like this, make sure you look at it from all sides and you’re not missing anything. And you’ll just throw it back in the water like this.
Stream Amphibians December 2016(docx) OR Stream Amphibians December 2016 (pdf)











