Ι The Box Turtles Project Ι The Lizards Project Ι The Semi-aquatic Turtles Project Ι The Snakes Project Ι The Stream Amphibians Project Ι
The Ephemeral Pools Project
Before starting a project similar to the one described in this curriculum, contact your state wildlife resources commission or state division of fish and game to see what kinds of permits you need to work with animals.
This curriculum project focuses on the studies of seasonal wetlands called ephemeral pools that we conduct with our high school participants and teachers as a part of The HERP (Herpetology Education in Rural Places & Spaces) Project’s Herpetological Research Experience (HRE). This description of our curriculum provides ideas for conducting your own educational program and scientific investigation on temporary wetlands. As a part of this curriculum we provide information about ephemeral pools and describe how we conduct descriptive studies of various ephemeral pools in the North Carolina piedmont. We describe our sampling methods and techniques and our data collection and reporting procedures.
Introduction to Ephemeral Pool Project:
Ephemeral wetlands (temporary or seasonal pools) are abundant in rural areas of North Carolina’s piedmont in the spring and in the fall. Depressions in the ground with clay bottoms tend to hold rainwater and will sometimes be filled with overflow from nearby streams. Typically, these pools are shallow and are subjected to high levels of evaporation and transpiration from surrounding woody vegetation. These temporary ponds offer spotted and marbled salamanders breeding areas and a safe place to lay eggs. Both marbled and spotted salamanders are obligate species – as in obligated to breed in a place without fish – and their presence defines an ephemeral pool as such and distinguishes it from other wetlands like farm ponds that contain fish. We have many of these pools in our area of the country and have access to several of these pools on private property where we are able to set minnow traps and sample the organisms that live there using both minnow traps and aquatic dip nets. We have been studying several of the pools on our study sites for nearly a decade.



After completing this project, participants will be able to:
1. Distinguish salamander larvae from tadpoles (frog larvae)
2. Identify frogs and salamanders that frequent North Carolina piedmont ephemeral pools
3. Explain the life cycles of these frogs and salamanders
4. Demonstrate techniques to safely catch and handle frogs and salamanders
5. Explain the importance of these temporary pools as breeding grounds for frogs and salamanders
6. Describe the threats to ephemeral pool environments and their inhabitants
7. Explain that amphibians are good bio-indicators of environmental quality
While doing fieldwork in North Carolina, participants may encounter insects and other arthropods including chiggers, yellow jackets, ticks and spiders. Using insect repellant (but not on your hands if you plan to collect herps) and wearing a hat and long pants are useful ways of preventing these animals from biting you, stinging you or attaching to you. Pulling one’s socks over the bottoms of pants legs is an especially good way of preventing ticks, chiggers, and spiders from crawling up your legs. Participants should also wear sunscreen and carry water; sturdy boots are useful when hiking in rough terrain. If you are in an area where you may encounter snakes, know that feet and ankles are the most common bite locations, followed by hands. Wear protective footwear and long pants or gaiters and look before you place your hands down or around a tree. Always hike with a partner and let someone else know your itinerary.
When handling any amphibian, ALWAYS moisten your hands first with water to prevent the animal from drying out. Tap water from city reservoirs, often called ”city water”, or other chemically treated water (including bottled water) should NEVER be used to hydrate amphibians or wet hands. Amphibians, with their permeable skin, are much more sensitive to touch than scaly-skinned reptiles and must be kept wet while handling. Once captured, frogs and salamanders should be placed in a container with ephemeral pool water so that they remain in their habitat. Larvae of frogs are called tadpoles and, like salamander larvae, are especially fragile and should be handled as little as possible and with much care. Detailed handling information for various kinds of amphibians of different ages follows.
Compared to the temperature of a salamander, human hands are hot. The heat from hands and the stress of being handled can actually cause the death of a salamander. There are several ways to handle your amphibian with a minimum of stress (for both the animals and the humans). All sizes of adult salamanders should be confined in your hand with a firm but gentle grasp. Salamanders held in the palm of your hand will often jump to the ground and may injure themselves. For small salamanders, form a cage with your hands and fingers. This will allow air to move but will not allow the animal to escape. For medium and large size salamanders (those about 5 grams and larger), grasp them firmly but gently just behind the forelimbs or in the middle of the body between the forelimbs and hind limbs.
Larval and neotenic salamanders (neotenic salamanders are salamanders which are capable of reproducing in a larval form) should never be grasped around the head or neck, because the gills can be easily damaged. Larvae should not be grasped with bare hands, but may be scooped up in water with bare hands for momentary observation. Under most circumstances, observing these animals in this tender life-stage can be done while they remain in containers of ephemeral pool water. For close examinations, the larvae should be placed in a clear plastic bag or a shallow tray with a small amount of water that will allow them to swim freely. As much as possible, larvae should be examined only while they are in water.
When catching and handling frogs and toads remember that their skin, like salamanders, affords little protection against desiccation (drying) or against heat. Their thin skin absorbs oxygen from the water and the atmosphere, but the skin can also absorb anything else that it contacts. If you have perfume, insecticide, hand lotion, or even soap residue on your hands and you handle an amphibian, the frog or salamander will absorb whatever is on your skin, perhaps with fatal results.
Toads are quite easy to handle, but do so gently. They may jump out of your hands and fall a meter or more. When toads are caught and picked up they often urinate so expect to get peed on.
These animals must always be handled gingerly. This can be a problem with frogs since they have strong muscular legs that allow them to escape a captor’s hands. A good way to hold a frog without damage is to extend the legs and hold them gently but firmly while supporting the body of the frog with the other hand. As long as the frog cannot cock its legs, it will have a hard time jumping.
Below Are Three Different but Appropriate Methods for Handling Adult Frogs


 Avoid handling late stage tadpoles (i.e. large tadpoles with forelimbs already emerging and tail absorption underway). This is a critical developmental phase and amphibians are highly vulnerable at this time. Many individuals do not make the transformation. Release amphibians, and all other animals where they were caught and where they can quickly find cover. If releasing them under rocks, logs or coverboards, lay the structure down first and then let the amphibian safely crawl under it instead of putting the cover pieces on top of the animal.
Avoid handling late stage tadpoles (i.e. large tadpoles with forelimbs already emerging and tail absorption underway). This is a critical developmental phase and amphibians are highly vulnerable at this time. Many individuals do not make the transformation. Release amphibians, and all other animals where they were caught and where they can quickly find cover. If releasing them under rocks, logs or coverboards, lay the structure down first and then let the amphibian safely crawl under it instead of putting the cover pieces on top of the animal.
These wetland systems, ephemeral pools, support many species and are the sole breeding grounds for some species, such as certain species of Ambystoma (mole) salamanders.
Salamanders and other amphibians can make up the greatest vertebrate biomass in a woodland ecosystem and the loss of ephemeral pools could be detrimental to the entire ecological community (Best and Welch, 2014). Data collected can be analyzed and used in defense of the habitat to prevent habitat destruction, habitat fragmentation and development of habitat since having data on pools known to be successful breeding habitat makes for a stronger case against urban encroachment than ‘potential’ habitat. Documenting the presence and abundance of organisms in ephemeral pools is critical to making a scientifically sound case for protecting the local environment.
- GPS
- A caliper is a device used to measure the distance between two opposite sides of an object.
- Salamander sticks (Walston & Mullin, 2005); small diameter (1/4” – ½” pvc pipe), masking, duct or electrical tape & small piece of cardboard



 These four photographs show the sequence of putting a Newt in a Bag in a Salamander Stick and then Measuring the Total Length of the Newt with a Caliper.
These four photographs show the sequence of putting a Newt in a Bag in a Salamander Stick and then Measuring the Total Length of the Newt with a Caliper.
- Magnifying lenses
- Spring scales of various sizes (or digital scales)
- A spring scale is a spring fixed at one end with a hook to attach an object at the other.
- Thermometer
- pH strips
- Tape Measure (at least 100’ 0r 33 m)
- Aquatic dip nets
- Minnow traps (with one empty .5 liter soda/water/juice bottle & cap per trap)
- A Pictorial Key to Aquatic Insect Orders (we use printed and laminated copies of the North Carolina State Parks’ macroinvertebrate keys. These are included in this document after The Herp Project Data Sheet and are available for a free download):
http://www.learnnc.org/lp/media/uploads/2010/06/macrokey.pdf
http://www.ncparks.gov/Education/docs/lano_eele.pdf (on page 3.1.8 and 3.1.9) - A field guide to the amphibians local to your area (we use Amphibians & Reptiles of the Carolinas and Virginia (2010).)
Catching Vertebrates & Macroinvertebrates
Our minnow traps (with two small open funneled ends – see photo) are typically not baited. Organisms swim in but cannot swim out due to the shape of the trap. When setting the trap we insert a plastic bottle filled with air and capped to make the trap float. This allows organisms to surface for air as needed while in the trap. Traps are pulled onto land and gently tapped to move all animals to one end, and opened from a latch in the middle. Traps should be checked at least once every 24 hours. Checking traps at least one every 24 hours is critically important as minnow traps are active traps and animals that are caught in these traps cannot escape. We check our trap in the morning and remove them and then reset them about dark. We often catch tadpoles, salamanders, salamander larvae, frogs, invertebrates of all kinds and sometimes even a water snake.
How to check a minnow trap
Student checking a minnow trap
In addition to setting and retrieving minnow traps we also use aquatic dip nets for sampling.
Identifying Macroinvertebrates & Invertebrates
We start our biotic study at the pools by first identifying species. Participants decide if our animal is an invertebrate (insect, crustacean, etc.) or a vertebrate (salamander, frog, or snake). For organisms without backbones, invertebrates, we use pictorial keys (see information on our list of materials) and the common names of organisms, which frequent ephemeral pools. Macroinvertebrates are invertebrates that you can see with just your eyes; additional magnification is not needed although a hand lens makes them easier to see. Macroinvertebrates are identified to the family or genera level when possible.
Fairy shrimp (http://www.usgs.gov/blogs/csrp/2011/06/17/a-missouri-river-delicacy/an invertebrate) is an obligate species, common in the pools where we sample (although present in late winter/early spring) and are another indicator species, only reproducing in this environment. Other common invertebrates found in the Ephemeral Pools we identify include:
- Dragonfly nymphs (to the right)

- Backswimmers
- Water Boatman http://scioly.org/wiki/index.php/Water_Quality/Macroorganism_List#Water_Boatman
- Water scorpions http://scioly.org/wiki/index.php/File:Water_scorpion_ranatra_species.jpg
- Giant water bugs (to the right)

- Diving Beetles http://scioly.org/wiki/index.php/Water_Quality/Macroorganism_List#Predacious_Diving_Beetle
- Crayfish (to the right)

We identify vertebrates using Amphibians & Reptiles of the Carolinas and Virginia (2010), the field guide that we give each of our HRE participants. The field guide provides detailed information about the natural history of each species as well as range maps. In our project, salamanders and frogs are identified to species. The most common salamanders encountered in the NC piedmont are spotted salamanders, marbled salamanders and eastern newts (also a type of salamander). Spotted salamanders are winter breeders, moving into the pools with the first warm rains of the year. Their large, conspicuous egg masses are easily identified. Marbled salamanders breed in autumn, laying their eggs in small burrows in the mud, where they wait, with their mother’s body wrapped protectively around them, until the pools fill with water to hatch. The presence of salamander adults, egg masses, or salamander larvae is used to identify an ephemeral pool as such. See additional photos and range maps for salamanders and frogs at www.herpsofnc.org.
The herps below are commonly found in ephemeral pools and you can get additional information about each animal by going to the Herps of North Carolina website.
- Eastern (red spotted) Newts
- Spotted Salamanders
- Marbled salamanders

Female Marbled Salamander
Common species of frogs in and nearby NC Piedmont Ephemeral Pools include:
- Bullfrog (Lithobates catesbeiana) http://www.herpsofnc.org/herps_of_NC/anurans/Rancat/Ran_cat.html
- Green Frog (Lithobates clamitans) http://www.herpsofnc.org/herps_of_NC/anurans/Rancla/Ran_cla.html
- Pickerel Frog (Lithobates palustris) http://www.herpsofnc.org/herps_of_NC/anurans/Ranpal/Ran_pal.html
- Southern Leopard Frog (Lithobates sphenocephala) http://www.herpsofnc.org/herps_of_NC/anurans/Ransph/Ran_sph.html
- Chorus Frogs (Pseudacris feriarum) http://www.herpsofnc.org/herps_of_NC/anurans/Psefer/Pse_fer.html
- Cricket Frogs (Acris crepitans) http://www.herpsofnc.org/herps_of_NC/anurans/Acrcregry/Acr_cregry.html
- Copes Grey Tree Frogs (Hyla chrysoscelis) http://www.herpsofnc.org/herps_of_NC/anurans/Hylchrver/Hyl_chrver.html
- Spring Peeper (Pseudacris crucifer) http://www.herpsofnc.org/herps_of_NC/anurans/Psecru/Pse_cru.html
Holding and Identifying a Frog:
Threats to Ephemeral Pools and Threats to Inhabitants of Ephemeral Pools: Pollution run off can change the pH of the water and surrounding soil. Many ephemeral pools are drained as people believe that they are places where mosquitos breed. Pool owners may add fish for mosquito control but fish eat salamander eggs. Because we have lost so many of these important wetlands, environmental groups around the country are creating wetlands on school grounds, local park lands and elsewhere. You can make a depression that will fill with water in your own backyard and offer salamanders a place to breed.
Every location that is studied must have a protocol for accurate data collection. Abiotic data to be collected include:
- Exact location (GPS coordinates)
- pH
- Water temperature
- Air temperature
- Circumference of vernal pool & water depth at deepest spot
How to Measure Circumference of Ephemeral Pool
One of our ephemeral pools dries up nearly every summer. Another pool has never dried up completely but certainly becomes smaller as the summer days heat up. As pools dry out, animal inhabitants must find another place to live. If pools dry out too quickly animal losses may be greater since larval metamorphosis might not be able to keep pace with the rate of evaporation.
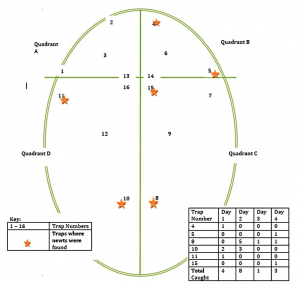
In addition to the general abiotic factors discussed above, careful records should be kept of all living organisms. Below is a sketch of an ephemeral pool we monitor. Note trap locations and data table.
Ephemeral Pool Map for CEM’s House 2009
Each trap should be emptied carefully and contents recorded. The interval between setting traps and checking traps (active traps such as minnow traps from which animals cannot escape), should be as short as possible, never more than 24 hours. A set trap is a potential death sentence for the animal to be trapped and it is the researcher’s ethical imperative to check traps in a timely manner. Lead instructors/investigators must make every effort to prevent trap deaths from exposure or drowning. The traps should be positioned using some type of flotation device so that captured amphibians without gills have access to air. We make sure that each of our traps floats before we leave our study site.
Our data includes identifications of various species of reptiles, amphibians and macroinvertebrates, distinguishing between males and females when possible, estimating ages, measuring and weighing animals, and using species-specific techniques to mark animals. Vertebrates are identified to life stage – tadpole or larva with/without legs, tails, gills as appropriate. Remember – larval forms are very delicate. The only data we collect on amphibian larvae are gills or no gills (salamanders) and how many and which legs are present and whether or not a tail (frogs) is still present. We do not take any measurements of amphibian larvae. Participants are typically fascinated by the real life lessons on metamorphosis. Data we collect on adult salamanders and frogs and toads include sex, life stage/age, length, and weight. Data sheets for this project are included in the Appendix.
A picture of a male eastern newt can be seen at http://www.wildlife.state.nh.us/Wildlife/Nongame/salamanders/east-redspot_newt.htm.
| Data | Sex(F or M) | Life stage/age | Total Length (mm)Adult Salamanders Only | Snout to Vent Length (SVL) (mm)Adults only | Mass(g)Adults only |
| Tips | Male or Female?Dimorphic ColorationExamples:1.) Male eastern newts have black “Velcro”-like pads or circles on the ends of their toes and the inside of their back legs2.) Marbled salamander males are more brightly colored than females3.) Male frogs have dark colored throats (which are deflated vocal sacs) – and only male frogs call (females do not have vocal sacs and do not call) | Adult or Larva?For Salamander larvae – Are gills present?How many and which legs are present (no legs, 2 front legs, or all 4 legs)?For Tadpoles – How many and which legs are present (no legs, 2 front legs, or all 4 legs)?Is there a tail? | What is the Length?Use calipers to measure.Measure from the tip of the snout to the end of the tail. | What is SVL?The vent is the opening between the two hind legs that is used for excretion & reproduction.Use calipers to measure.Measure from the tip of the snout to the posterior end of the vent. | What is the Mass?Place the organism in a plastic bag with a small amount of ephemeral pool water.Use a spring scale to weigh the organism and the bag.Once recorded, remove the organism from the bag gently and reweigh the bag by itself.Subtract the mass of the bag from the mass with the organism to get the mass of the organism. |
| These two lengths are important as salamanders can lose and regenerate their tails.Find the ratio of the two lengths by dividing the SVL by the total length. | |||||
How to mass a salamander – https://vimeo.com/112870042
Additional Research Possibilities:
1) Frog Loggers: We use Frog Loggers to record frog calls at the ephemeral pools that we study. Frog loggers are digital recording devices (data is stored on SD cards) that can be programmed to record sounds at specific times of the day and with a specific regularity. HRE participants and teachers then listen to these recorded frog calls and ask such questions as: 1. What frogs are calling at Matthews’ ephemeral pool? 2. Are these the same frogs that are calling at CCR’s ephemeral pool? 3. If the same [or different] calls are heard, why do you think this is so? 4. Are there seasonal patterns in frog calls? 5. Are there daily patterns in frog calls?
If you need to learn North Carolina frog calls you can easily do so at www.herpsofnc.org, a Davidson College website on reptiles and amphibians of North Carolina. Information and range maps are provided about each species of frog in the state and frog calls can be heard by clicking a play button. Other states may have similar frog call databases.
2) Fluorescent Tracking Powder and Blacklights: Fluorescent tracking powder can be used to analyze the terrestrial movements of salamanders at night. Simply dip the salamanders’ feet in the powder and release it at the point of capture, being careful not to get any of the powder in the salamanders’ mouth or eyes. Return in the evening with a blacklight to follow its path. Identifying where the salamander was first spotted and measuring how far the salamander traveled can provide further data for analysis.
3) Making observations of salamander behavior in a science notebook enhances students’ observation skills.
Through use of the free Herp Project android application (available for FREE download:http://theherpproject.uncg.edu/apps-collecting-data/), HRE participants record data and upload it to an open source database found on the Herp Project website (http://nc-herps.appspot.com/). This enables us to compare our data with previous years, and we can download data sets for further analysis. We also report our data to the Carolina Herp Atlas (www.carolinaherpatlas.org), a citizen science database initiated to document the distributions of amphibians and reptiles across North and South Carolina.
This project is supported by the National Science Foundation, Grant No. DRL-1114558. Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Articles
Best, M. & Welsh, Jr., H. (2014). The trophic role of a forest salamander: impacts on invertebrates, leaf litter retention, and the humification process. Ecosphere (5) 2 Article 16. (Retrieved from http://www.esajournals.org/action/showCitFormats?doi=10.1890%2FES13-00302.1.
Walston. L. & Mullin, S. (2005). Evaluation of a New Method for Measuring Salamanders. Herpetological Review 36 (3), 290 – 292.
Books
Beane, J., Braswell, A., Mitchell, J., Palmer, W. & Harrison III, J. (2010). Amphibians & Reptiles of the Carolinas and Virginia. (2d Ed. Revised). Chapel Hill, NC: UNC Press.
Calhoun, A. J. K. & deMaynadier, P. G. (2008). Science and Conservation of Vernal Pools in Northeastern North America. Boca Raton, FL: Taylor & Francis.
Web Resources
Climate Change and Ephemeral Pool Ecosystems: Potholes and vernal pools as potential indicator systems.
http://geochange.er.usgs.gov/sw/impacts/biology/vernal/
Checklist of the amphibians and reptiles of North Carolina. Beane, J. C., and A. L. Braswell, 2011.
http://naturalsciences.org/research-collections/research-specialties/amphibiansreptiles
Winter and Early Spring Vernal Pool Egg Mass Identification: Pt. 1 Frogs
http://midatlanticnature.blogspot.com/2012/01/winter-and-early-spring-vernal-pool-egg.html
Winter and Early Spring Vernal Pool Egg Mass Identification: Pt. 2 Salamanders
http://midatlanticnature.blogspot.com/2012/02/winter-and-early-spring-vernal-pool-egg.html
Transcripts of Videos
Transcript Introduction to Ephemeral Pool Project
Kathy: I’m Dr. Kathy Matthews. I’m on the faculty at the University of North Carolina at Greensboro and I’m the PI, the Principle Investigator, on the HERP Project: Herpetology Education in Rural Places and Spaces, and all of you are down at my vernal pool today, so in just a few minutes we’re going to do a vernal pool exploration.
Transcript How to Check a Minnow Trap
Leader: See, just pretend it’s a fountain and dump them back in, you wanna just put it in the water and dump everything out.
So when we pull a trap, as I said yesterday, they are minnow traps and they have holes on either side and they have the bottle in it so it was able to float. So, we’re going to pull this first one in. And it has a, most of them have a number on it. (Pulling in the trap by a string from the water.) So what we’re going to do is… this is trap 44 so the boys are going to record 44. And then you’re going to tip it up to one side and knock it so that everything comes down to one side. And this is like a little pin, they can get tricky. You got to push it in and it all comes out. (Opens trap to examine contents.)
So I’m just gonna double check the top that I pull off to make sure there’s nothing in there cause we don’t want any animals to die, and then I take the bottle out. Then what I’m going to do, is, I’m going to go through to see what is in here.
Transcript Student Checking Minnow Trap
Kathy: Yeah, right now it would totally murk up the pool so, yeah, thanks for being so careful walking through there with the waiters.
(Student opens trap, hands the top to another student to check, takes the bottle out of the trap, and observes the bottom of the trap.)
Student: Oohh, look at that! (Student shakes a tadpole out of the trap.)
Student: Yeah, that was a good one. So there were three giant tadpoles in that and one smaller tadpole.
Transcript of Holding and Identifying a Frog:
Andy: Ok, as I’ve said before, I find the easiest way to hold frogs is by the very end of their rear, or hind, legs so that they can’t cock to jump. So, what we want to do is identify this so everybody get their field guides out and we’re going to look for the characters that we discussed earlier and y’all are going to tell me what I’m looking at here.
Participant: Well, you’ve got that lateral, or ridge, what’s it called again?
Andy: Lateral line, lateral fold.
Participant: That goes down behind its ears.
Andy: Behind its ears. There’s one clue.
Erika: Do you remember what it’s called?
Participant: Tympanum?
Erika: Tympanum, yes!
Andy: Ok, so, do we see any brightish or neon green up under its eye. So what do you think, a frog this big, what do you think our two choices are?
Participants: Green frog or Bullfrog.
Andy: Green frog or Bull so do we see that neon green under the eyes that Green frogs have? Now Bulls can sometimes look like that too.
Participant: Not really.
Andy: I don’t really see that. It’s kinda green but not really neon green.
Participant: Um, 145 and 146.
Andy: So, the real key in this case is what that fold does. And what have we decided it does?
Participant: Curves back around behind the ear so I say it’s an American Bullfrog.
Andy: There you go. And let’s look at his stomach. So you see the splotching, the darker splotching on the venter, right? Ok, so that pretty much tells us. Ok, so now let’s look at the size of the tympanum and y’all tell me whether we’re looking at a young man or a young lady here.
Participants: Female. They’re small in size.
Andy: Yeah, smaller tympanum. Ok, so, anyone want to hold the frog?
Erika: Let’s show them the eyeball. Ok, look at his eyes, guys. See, I can push it back, see how I can just push it down in?
Participant: Does that…
Andy: Oh they have muscles that can actually pull their eyeballs in and out. So why would you want your eyeballs up like that?
Participants: So you can see above the water?
Andy: Yeah, so you can stay in the water but see what’s above the water. But why would you want to be able to pull them down?
Participants: So predators don’t see you.
Andy: Predator… let’s think about some other things. How do you reckon frogs find a lot of their food?
Participants: Under the water… Oh so they can sneak up on them.
Andy: Well, they’re gonna be nosing around in litter right? Pushing through leaves and sticks and twigs. Do you want your eyes up where they can be damaged doin that kind of thing?
Participant: Uh uh.
Andy: No, you want to pull them down. And most amphibians depend on their sense of smell as their primary sense. Ah, I work on salamanders and I had a salamander physiologist from Germany once tell me that salamanders are just giant noses. They can see but they don’t use their eyes much. And salamanders can do the exact same thing, they have telescope eyes that they can pull down when they’re foraging under leaves or under rocks in streams and things like that.
Participant: May I hold him?
Andy: Sure, so just grab right there at the end. There you go.
Participant: Oh, ok. I thought he would be more jumpy.
Andy: No, you see, if he can’t cock his legs, there’s not much he can do. Or she, excuse me, that’s a young lady.
Participant: There were three tadpoles, correct?
Participant: Four. Four small ones.
Erika: You want to try picking one up and you can see what it is?
Participant: Any of them have legs?
Andy: Do you think Green frog adults would eat Bullfrog tadpoles?
Participant: Sure.
Andy: Sure. Would an adult Bullfrog eat a Green frog or a Green frog tadpole? Sure.
Transcript How to Measure Circumference of an Emphemeral Pool
Leader: So what we’re going to do, we’re going to work together first and we’re going to measure the circumference of the ephemeral pool. So, how do you think we’re going to start this?
Participant: You go across.
Leader: Ok, we could go all the way across but keep in mind we haven’t pulled any traps. If we go all the way across the water’s going to be even murkier and it could harm some of the species that are in there.
Participant: Like, maybe go around it?
Leader: Right, right. So, do you want to start?
Participant: Yes.
Leader: And you guys can follow. So Tory is going to be the leader for this. And I’ll give you a hint, get a stick that you can put through here, and that way it can be your starting place. Ok? And you can go either to the right or to the left. Jacob and I will follow you along.
Right at the edge of where the water is and then we’re just gonna use the sticks.
(Students are walking around the edge of the ephemeral pool with a tape measure, making sure that the tape is secured by sticks along the way.)
Leader: Ok, so we’ve only come this far around, what do you think we’re gonna, how are we gonna… How are we gonna finish? Yep, roll it back up, so if someone could go to where we started and take it off the stick but put the stick back where we started. And we can start right here and roll it. So if you girls wanna tag team it, you can do that.
Ok, do you see where the stick is, where we left off? So if one of you can, yeah, awesome.
Participant: 85 and 1 ½
Leader: Ok, so, we have, the second time we have 85 with 1 ½ inches and we have to add it to?
Participant: 100.
Leader: So, 185 feet. And then roughly two inches, so we’re going to record that. And then we’re going to notice how it changes throughout the week based on the weather, ok?
So let’s roll that back up and we’ll get our data sheets out and record that.
Transcript How to Mass a Salamander
Leader: We’re going to measure the bag with the water in it, and then we measure it with the newt in it, then we subtract the two and we’ll find the measurement, ah, the mass of the organism.
(Student puts newt in a ziplock baggie with some water in it then attaches a spring scale to the bag.)
Leader: So 7.8 subtract 3.9.
If you think you have an ephemeral pool on your property or if you know someone else who has an ephemeral pool on their property, a landowner packet can be used to notify landowners of the special habitat on their property and offers suggestions for how they can help maintain it. The packet raises awareness about the habitat and the species that use this habitat. Nearly all of our ephemeral pools are on private land and therefore landowner support is critical. A landowner packet may be downloaded below.
Ephemeral Pools with Lacey table Jan 2017(docx) OR Ephemeral Pools with Lacey table Jan 2017 (pdf)